

43992
meddg
Enterprise Blockchain for Clinical Trials
Switzerland
Market: Medicine, Pharmacology, Other, Blockchain
Stage of the project: Prototype or product is ready
Date of last change: 10.03.2020
Switzerland
Market: Medicine, Pharmacology, Other, Blockchain
Stage of the project: Prototype or product is ready
Date of last change: 10.03.2020
Idea
Blockchain Use Case for Integration and consolidation of distributed stakeholders in healthcare ecosystem with patient data
Current Status
PoC for the patient recruitment and eConsent for two different frameworks are ready, the next steps are the trial protocol integration and clinical monitoring development.
We paeticipate at the Transcelerate Hackacton, which is the most prominent platform for digital healthcare solutions
We paeticipate at the Transcelerate Hackacton, which is the most prominent platform for digital healthcare solutions
Market
Patient recruitment companies, and sponsor companies, like big pharma, middle and small sized in vitro vascular Medtech solution providers,
in EU, CH, USA, UK and other OECD countries with a national Health Insurance system
in EU, CH, USA, UK and other OECD countries with a national Health Insurance system
Problem or Opportunity
For these steps to complete the clinical trial process for different phases (1-4) we need a established domain expert and development team. The opportunity is the service offer mainly for patient recruitment companies, and sponsor companies, like big pharma, middle and small sized in vitro vascular Medtech solution providers.
The challenge is the cultural change for a new technology. And that is again the opportunity to boost the process acceleration time2market, save efforts, and higher FDA compliance, security and integration.
The challenge is the cultural change for a new technology. And that is again the opportunity to boost the process acceleration time2market, save efforts, and higher FDA compliance, security and integration.
Solution (product or service)
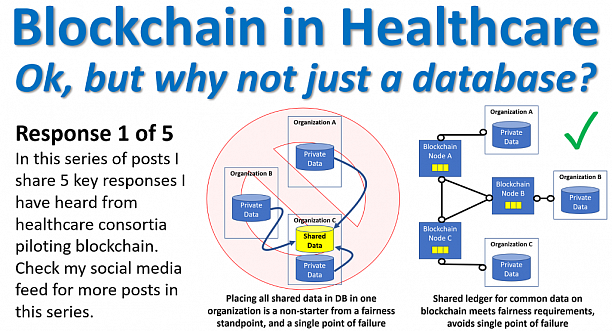
Cloud Platform for clinical trial process flow, in which 12% the artifacts lost in a distributed and highly regulated stakeholder landscape. The interoperability is an issue, and data is not governed, in the world of GDPR and FDA.
We make this happen with our solution, completely structured instances for untrusthing ledgers in the workflow; which is secure, traceable, personal data compliant, patient centric, immutable and transaprent.
First part of service is the patient recruitment (consent is the integrated part), which is already a big quality improvement.
We make this happen with our solution, completely structured instances for untrusthing ledgers in the workflow; which is secure, traceable, personal data compliant, patient centric, immutable and transaprent.
First part of service is the patient recruitment (consent is the integrated part), which is already a big quality improvement.
Competitors
Two competitors, one in India, one in UK. Both have less advanced technology and slower than our solution, No in EU and CH
Advantages or differentiators
Pharma companies:
Not only saving in efforts, savings to Time2market
Tracibility for compliance with documents, evidences, compliance
Big advantage for the small and middle sized pharma providers
Reliable studies
Patient:
Drugs faster in the market, Solutions with quality results, side effects known
Concent and privacy - patient centric
Regulatory bodies:
Democracy and innovation to the new therapies
Not only saving in efforts, savings to Time2market
Tracibility for compliance with documents, evidences, compliance
Big advantage for the small and middle sized pharma providers
Reliable studies
Patient:
Drugs faster in the market, Solutions with quality results, side effects known
Concent and privacy - patient centric
Regulatory bodies:
Democracy and innovation to the new therapies
Finance
This is a R&D project, there are local and Swiss Government Funds, we are in the application process.
Business model
Integration and accelaration of patient recruitment process for clinical trials, main process for pharma products market entry
Money will be spent on
Basicly, Development, Infrastructure, Admin & Marketing, Patent, Sales, Interest and Taxes, a cash flow and earnings statement will be presented in the pitch
Team or Management
Risks
Pharma companies:
Culture - change to negotiation and agreement from small and mine
Need for consolidation - resistance to change
Comfort zone - still
Clinical Research Org:
Revenue cut
Culture - change to negotiation and agreement from small and mine
Need for consolidation - resistance to change
Comfort zone - still
Clinical Research Org:
Revenue cut
Photos
Presentation
Sign in/Sign up