

53373
Phonolyser Oy
The Heart Speaks Better with Phonolyser™
Finland
Market: Medicine, Other, Artificial Intelligence
Stage of the project: Prototype or product is ready
Date of last change: 16.08.2021
Finland
Market: Medicine, Other, Artificial Intelligence
Stage of the project: Prototype or product is ready
Date of last change: 16.08.2021
Idea
Phonolyser™ is a smart heart analyzer to help pediatricians and healthcare staff to differentiate innocent murmurs from pathologic ones so-called Congenital Heart Disease.
Current Status
We are having a working product and sold 15 devices.
Our current customers are:
● Hospitals
● Clinics
● Diagnostic Centers / Healthcare Centers (Public & Private)
● Pediatricians
● University hospitals
Our current customers are:
● Hospitals
● Clinics
● Diagnostic Centers / Healthcare Centers (Public & Private)
● Pediatricians
● University hospitals
Market
University Hospitals
Clinics
Healthcare Centers (Public & Private)
Pediatrics
US market: Insurance companies
Clinics
Healthcare Centers (Public & Private)
Pediatrics
US market: Insurance companies
Problem or Opportunity
Detecting and diagnosing congenital heart disease at early stages.
The Global Burden of Congenital Heart Disease is a problem with the heart's structure that exists since birth. Simply means the blood that flows through the heart is not normal
CHD kills 2 times more children each year than all forms of childhood cancer combined.
Current process:
Approach No.1
Stethoscope (Examination tool)
ECG (Examination tool)
Pulse-Oximetry (Examination tool)
They are available but not designed for proper diagnosis
Approach No.2
Echocardiogram (CHD diagnosis tool)
MRI (CHD diagnosis tool)
Rarely available, Expensive, and not easily accessible
Phonolyser:
Patented technology, detects, records, and displays heart murmurs in newborn babies faster, better and cheaper.
Better: Easy to operate; Healthcare staff of all levels, with few days of training, can use it
Faster: Patient examination takes less than 3 minutes
Cheaper: The most affordable and accurate device for diagnosing CHD in the market
Packed with technologies including AI, Doppler, sound effects, and ECG to accurately diagnose Congenital Heart Disease
Portable diagnostic device
The Global Burden of Congenital Heart Disease is a problem with the heart's structure that exists since birth. Simply means the blood that flows through the heart is not normal
CHD kills 2 times more children each year than all forms of childhood cancer combined.
Current process:
Approach No.1
Stethoscope (Examination tool)
ECG (Examination tool)
Pulse-Oximetry (Examination tool)
They are available but not designed for proper diagnosis
Approach No.2
Echocardiogram (CHD diagnosis tool)
MRI (CHD diagnosis tool)
Rarely available, Expensive, and not easily accessible
Phonolyser:
Patented technology, detects, records, and displays heart murmurs in newborn babies faster, better and cheaper.
Better: Easy to operate; Healthcare staff of all levels, with few days of training, can use it
Faster: Patient examination takes less than 3 minutes
Cheaper: The most affordable and accurate device for diagnosing CHD in the market
Packed with technologies including AI, Doppler, sound effects, and ECG to accurately diagnose Congenital Heart Disease
Portable diagnostic device
Solution (product or service)
Phonolyser™ is a smart heart analyzer to help pediatricians and healthcare staff to differentiate innocent murmurs from pathologic ones so-called Congenital Heart Disease.
More than 2000 patients have been diagnosed with Phonolyser.
More than 2000 patients have been diagnosed with Phonolyser.
Competitors
Indirect competitors:
MRI and Echocardiography devices. Brands like Siemens, GE, Toshiba, Philips and some more
Approach No.1
Stethoscope (Examination tool)
ECG (Examination tool)
Pulse-Oximetry (Examination tool)
They are available but not designed for proper diagnosis
Approach No.2
Echocardiogram (CHD diagnosis tool)
MRI (CHD diagnosis tool)
Rarely available, Expensive, and not easily accessible
MRI and Echocardiography devices. Brands like Siemens, GE, Toshiba, Philips and some more
Approach No.1
Stethoscope (Examination tool)
ECG (Examination tool)
Pulse-Oximetry (Examination tool)
They are available but not designed for proper diagnosis
Approach No.2
Echocardiogram (CHD diagnosis tool)
MRI (CHD diagnosis tool)
Rarely available, Expensive, and not easily accessible
Advantages or differentiators
Has been tested on more than 2000 patients
98% accuracy
Patented technology, detects, records, and displays heart murmurs in newborn babies Faster, Better and Cheaper.
Better: Easy to operate; Healthcare staff of all levels, with few days of training, can use it
Faster: Patient examination takes less than 3 minutes
Cheaper: The most affordable and accurate device for diagnosing CHD in the market
Realtime OS and real-time results. No more human interpretation needed
98% accuracy
Patented technology, detects, records, and displays heart murmurs in newborn babies Faster, Better and Cheaper.
Better: Easy to operate; Healthcare staff of all levels, with few days of training, can use it
Faster: Patient examination takes less than 3 minutes
Cheaper: The most affordable and accurate device for diagnosing CHD in the market
Realtime OS and real-time results. No more human interpretation needed
Finance
Revenue model
As a diagnostic device:
Direct Sales:13 devices sold to Clinics and Pediatricians
Regional Distributors
Europe
East-Asia
Middle-east
Local Distributors
Vietnam (Negotiating)
India (Negotiating)
As an educational device
Direct sales
2 devices sold to university hospitals
Regional Distributors
Nordic
East-Asia
Local Distributors
Vietnam
We are testing the possibilities of:
Product as service / Per case / Per visit
Software update
Growth Plan
Data analysis (UN)
Regional and Country CHD analysis
Mainstream patient screening
Renal artery stenosis
Abdominal emergencies
Point of care (connecting through the mobile platform
Cost Structure
Team Salary and administrative overhead costs
CE marking, FDA, TGA, and some more certificates and marking
Product development and manufacturing – Continues development – R&D
Equipment – Software - Hardware
Marketing
As a diagnostic device:
Direct Sales:13 devices sold to Clinics and Pediatricians
Regional Distributors
Europe
East-Asia
Middle-east
Local Distributors
Vietnam (Negotiating)
India (Negotiating)
As an educational device
Direct sales
2 devices sold to university hospitals
Regional Distributors
Nordic
East-Asia
Local Distributors
Vietnam
We are testing the possibilities of:
Product as service / Per case / Per visit
Software update
Growth Plan
Data analysis (UN)
Regional and Country CHD analysis
Mainstream patient screening
Renal artery stenosis
Abdominal emergencies
Point of care (connecting through the mobile platform
Cost Structure
Team Salary and administrative overhead costs
CE marking, FDA, TGA, and some more certificates and marking
Product development and manufacturing – Continues development – R&D
Equipment – Software - Hardware
Marketing
Business model
Business model:
Direct selling the device: Diagnostic or educational
A device as a service (Testing in Finland and nordic)
DIstribution partners
Channels:
Distributorship
Direct selling
Representative
Youtube
Website
LinkedIn
The growth plan
Data analysis (UN)
Regional and Country CHD analysis
Mainstream patient screening
Renal artery stenosis
Abdominal emergencies
Point of care (connecting through the mobile platform)
Metrics:
Number of lives saved due to earlier detection
Number of hospitals, healthcare centers purchasing
Number of doctors using the device
Number of sales per month
Number of Faulty devices
Number of products delivered Ontime
Number of products in surplus inventory
Cost of goods sold
Operating margin
Gross Profit
Direct selling the device: Diagnostic or educational
A device as a service (Testing in Finland and nordic)
DIstribution partners
Channels:
Distributorship
Direct selling
Representative
Youtube
Website
The growth plan
Data analysis (UN)
Regional and Country CHD analysis
Mainstream patient screening
Renal artery stenosis
Abdominal emergencies
Point of care (connecting through the mobile platform)
Metrics:
Number of lives saved due to earlier detection
Number of hospitals, healthcare centers purchasing
Number of doctors using the device
Number of sales per month
Number of Faulty devices
Number of products delivered Ontime
Number of products in surplus inventory
Cost of goods sold
Operating margin
Gross Profit
Money will be spent on
CE Marking (Mid-2022) – US patent
ESC congress, EAP congress, Slush, Medica, Arab Health,….. (2021-2022)
Clinical trial in Finland, Clinical trial in Nordic countries
Testbed in targeted countries
Building and distributing 1000 devices (Jan 2022)
Continuous development in design and tech (Version 4.0)
Team building (Sales, Marketing, R&D)
Working with university hospitals in Finland, Nordic countries, and middle-east region
Testing and Selling to Vietnam (Partner is already in place)
ESC congress, EAP congress, Slush, Medica, Arab Health,….. (2021-2022)
Clinical trial in Finland, Clinical trial in Nordic countries
Testbed in targeted countries
Building and distributing 1000 devices (Jan 2022)
Continuous development in design and tech (Version 4.0)
Team building (Sales, Marketing, R&D)
Working with university hospitals in Finland, Nordic countries, and middle-east region
Testing and Selling to Vietnam (Partner is already in place)
Offer for investor
We are looking for the right partner who wants to be with us to solve the global burden of CHD which massively causing children's and newborn death every day even in 2021.
We will negotiate the details in the meeting.
We will negotiate the details in the meeting.
Team or Management
Risks
Losing time to market. We should be in the targeted market before mid-2022.
Copy the device by manufacturers. The AI part needs at least a year to reach our level of processing.
Risk of selling the device as plan due to Covid situation. We are facing delays in Vietnam right now.
Copy the device by manufacturers. The AI part needs at least a year to reach our level of processing.
Risk of selling the device as plan due to Covid situation. We are facing delays in Vietnam right now.
Incubation/Acceleration programs accomplishment
Chosen by Health Incubator Helsinki batch No.2 amongst more than 100 startups. Officially started in June 2021.
Won the competition and other awards
Winner of the pitching competition in Upgraded - health startup association of Finland
Chosen by Health Incubator Helsinki batch No.2 amongst more than 100 startups.
Chosen by Health Incubator Helsinki batch No.2 amongst more than 100 startups.
Invention/Patent
We are in the process of filing patents for Europe and the US.
The device has been patented locally.
The device has been patented locally.
Photos
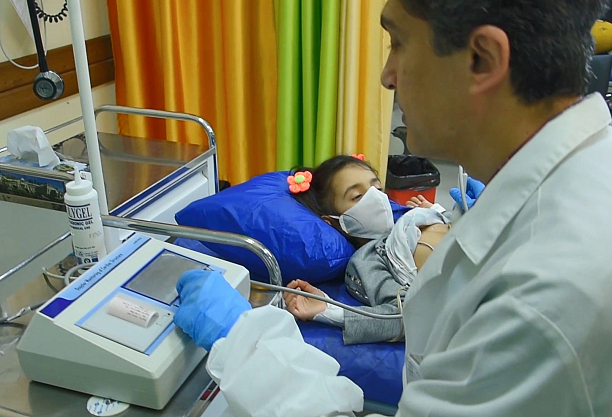

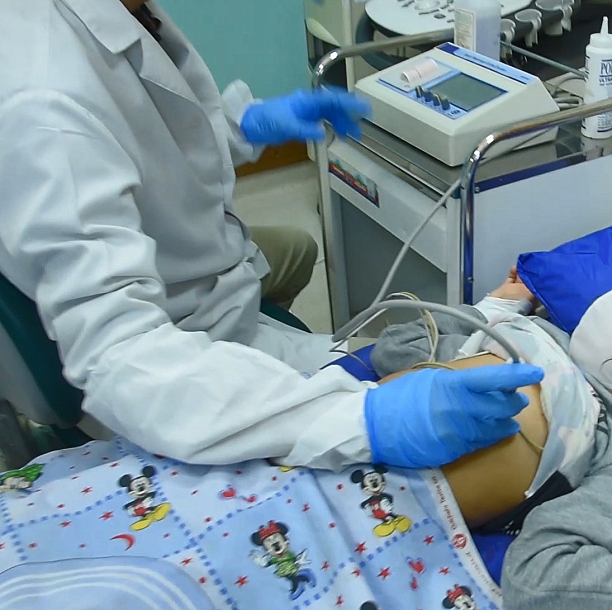
Product Video
Presentation
Sign in/Sign up